An encouraging first study: mortality rate down
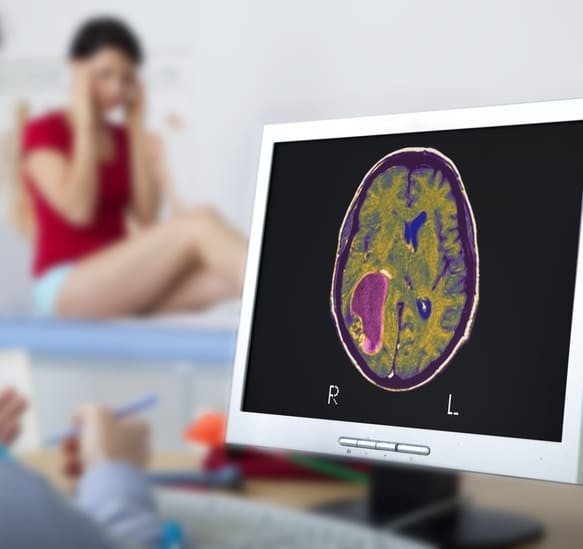
This type of clinical trial relies on a novel approach that involves administering a mouth spray of two molecules found in cannabis to measure its effects on disease-causing gliomas.
This trial follows a phase I study, conducted and monitored by the clinical trials unit, the Birmingham Cancer Research UK Centre, in the UK. It focused on observing the health outcomes of 21 sick patients who would have been treated, some with temozolomide (an anti-tumor agent) combined with Sativex (containing equal parts THC and CBD) and others with temozolomide and a placebo. It had revealed that 83% of patients treated with THC and CBD survived for at least a year, compared with 53% of patients in the placebo group.
Such promising results from this preliminary study prompted scientists to ask whether adding Sativex to chemotherapy treatment could prolong the lives of brain cancer patients, while improving their quality of life.
CBD and THC-based spray tested on patients with recurrent glioblastoma in trial phase
For the phase II trial, funded by the Brain Tumor Charity, researchers now aim to involve 232 glioblastoma (GBM) patients across 15 UK hospitals via enrollment set to begin in 2022. It is planned that two-thirds of the selected group will receive temozolomide and Sativex, as opposed to the remaining third who will receive only the antitumor agent and a placebo. Patients will be required to self-administer approximately twelve daily sprays of the mouth spray, for up to three years.
However, the said association also said that this would depend on the outcome of the appeal to help cover the £450,000 of expenses expected to be incurred in pursuing this new trial. Having lost 25% of its income since the start of the health crisis, the Brain Tumor Charity has been forced to block its usual grant program.